Kb Of Ba Oh 2
What is Barium Hydroxide?
Barium hydroxide is as well chosen as baryta with the formula Ba(OH)ii . Information technology is a clear white pulverisation with no odour. Information technology is poisonous in nature. It is ionic in nature for example, Ba(OH)2 (barium hydroxide) in aqueous solution tin can provide two hydroxide ions per molecule. Barium hydroxide is the just reagent described for metalizing carboxamidesBarium hydroxide was less degradative equally compared to barium oxide.
Other proper noun – Caustic baryta, Barium dihydroxide, barium(2+) dihydroxide
| Ba(OH)2 | Barium Hydroxide |
| Density | 3.74 chiliad/cm³ |
| Molecular Weight/ Molar Mass | 171.34 g/mol |
| Boiling Bespeak | 780 °C |
| Melting Point | 78 °C |
| Chemical Formula | BaH2O2 |
Table of Contents
-
- Barium hydroxide Structure-Ba(OH)2
- Physical Properties of Barium hydroxide-Ba(OH)2
- Use of Barium hydroxide-Ba(OH)two
- Health Hazard
- Frequently Asked Questions–FAQs
Barium hydroxide Structure – Ba(OH)ii
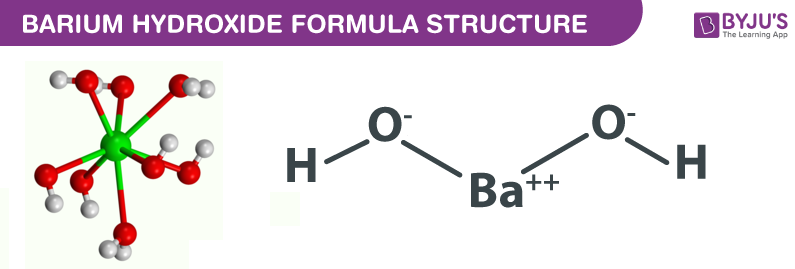
Physical Properties of Barium hydroxide – Ba(OH)2
| Odour | Odourless |
| Advent | White solid |
| Covalently-Bonded Unit | 3 |
| Vapour Pressure level | 0.48 kPa at 17.6 deg C |
| pH | 11.27 |
| Solubility | Slightly soluble in cold water |
Uses of Barium hydroxide – Ba(OH)2
-
-
-
- Barium hydroxide forms a strong caustic base of operations in aqueous solution. It has many uses, eastward.g., as a examination for sulphides; in pesticides; in the manufacture of alkali and glass.
- Utilise of barium hydroxide lime rather than soda lime, loftier sevoflurane concentration, high absorbent temperature, and fresh absorbent use.
- Used in the industry of alkalis, glass, oil and grease additives, barium soaps, and other barium compounds.
-
-
Health Adventure
-
-
-
- Inhalation of barium dusts can crusade irritation of the nose and upper respiratory tract and may produce benign pneumoconiosis known every bit baritosis.
- Barium ions are toxic to muscles especially heart, producing stimulation and and then paralysis.
- Information technology is extremely dangerous neurotoxin. Adverse result may result similar effects on the heart and the part of the key nervous organisation (CNS).
-
-
Ofttimes Asked Questions
What are the uses of barium hydroxide?
Industrially, barium hydroxide is used as a precursor to several other barium compounds. The monohydrate of this compound is widely used to dehydrate and extract sulfate from different products. The very low solubility of barium sulfate is utilized in this application.
How is barium hydroxide prepared?
Barium hydroxide is usually prepared by dissolving barium oxide (chemical formula: BaO) in h2o. The chemical equation for this reaction is provided beneath.
BaO + 9 H2O → Ba(OH)2·8H2O
It can be noted that this compound crystallizes into the octahydrate form, which is then converted into a monohydrate by heating it in air.
What happens when barium hydroxide is heated?
When heated to 800° C, barium hydroxide decomposes to yield barium oxide. Barium carbonate is provided by reaction with carbon dioxide. The strongly alkaline, aqueous solution undergoes neutralization reactions with acids.
Kb Of Ba Oh 2,
Source: https://byjus.com/chemistry/barium-hydroxide/
Posted by: garnerlifivend1962.blogspot.com


0 Response to "Kb Of Ba Oh 2"
Post a Comment